1st General Lyceum of Ilion

“Preparing for life in the 21st century”
Lesson plan in Sciences-physics
Grade B
Time needed: 1 hour
Teacher:mr Anestis Karapanagiotis
Thermodynamics – Gas laws
Purpose of this exercise:
Familiarity with the sizes: temperature, pressure and volume
Comprehension of the ways that the gasses change (isothermal – ηζότωρε)
How can the sizes (temperature, pressure and volume) be related to each other
according to the Gas laws
Representation of a diagram with the results. So we can understand better the
meanings of the diagrams and the relation of the sizes P, V, T.
Theory:
During the Reversible Process of a specific amount of an ideal gas, the volume
remains stable but the pressure and the temperature change proportionally:
P/T = stable
When the temperature remains stable during the Reversible Process of a specific
amount of an ideal gas (ηζόζερκε κεηαβοιή), the pressure and the volume
change inversely:
P*V= stable
In every alteration of a specific amount of an ideal gas it stands that:
P*V/T= stable
Generally:
 Inside the dispenser which is around the gas, there is an amount of water with a
Inside the dispenser which is around the gas, there is an amount of water with a
specific temperature ζ. We fill the dispenser with 300mL αηκοζθαηρηθού αέρα.
And we take for granted that the pressure of the atmosphere is:
Patm=1Atm=10^5 Pa.
The manometer shows the reading zero. We increase the pressure inside the dispenser at Δp, by moving the bucket. The manometer shows the reading which corresponds with the
change of the pressure Δp, so the total pressure of the gas inside the dispenser is: P=Patm+Δp
With the help of a thermometer, we measure the temperature of thewater, so the temperature inside the dispenser would be the same too. We
change it into absolute temperature (scale Kelvin)
Now we want to change the temperature of the water which is inside the dispenser, so we put outthe warm water with the help of output supply and we fill the dispenser with cold
water.
Necessary devices: A gas laws’ device GLA01
Notes for appliance instruments
We put a moving bucket inside the cylindric, metallic, 300mL volume cabin which has its one side closed. The bucket moves with help of a manual mechanism. Also there is a clasp which can recall the bucket.
2. Metallic manometer with a scale of 0 to 2.5 bar and lines every 0.02 bar. On the manometer a flexible conduit is adapted for its connection with a metallic chamber via a suitable stopcock of three entries. We mind that the small tube of the manometer is firmly screwed in order not to have a change in the sign (indication) because of a leak. We do not clench it overly otherwise it will break.
3. Digital multimeter/ thermometer with a sensor of temperature
This multimeter, for reasons of battery saving, turns off automatically after a few seconds of operation. For resetting the indication press the button POWER twice. The plug of the wire that is connected with the digital thermometer has two clamps of different width. Place it in a way that the wider clamp is in the same side with that of the screen. The plug of the ζερκοδεσγοσς , when we fix it to the metallic conduit and we put it on or we remove it from the cylindrical σδαηοιοσηρο , we mind that it passes through the nick to avoid its being worn.
4. Graduated scale from 0-36 mL with marks in every 2 mL, that refers to the air trapped in
the chamber at every phase of the experiment.
5. Stopcock with three entries.
6. Cylindrical container from PVC in which inside a metallic chamber is located for the creation of varying conditions of temperature. The container that receives water has a corner tube adapted close to its upper seal for its filling with water and below, close to its bottom, a flexible runoff tube for the disgorgement of the tube. We mind that there is not a leak of water at the bottom of the container that receives water from PVC . If there is a leak , we can exude at this point a drop of instant glue and let it drain.
INISPENSABLE KNOWLEDGE
Boyle’s law
Experimental procedure (from the guide for the use of the device for the laws of gases
GLA01)
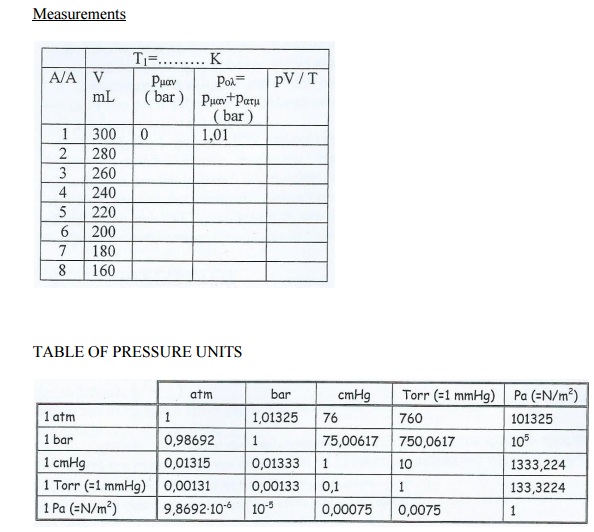
1. Turn the settings of the three swivels in such a position that they allow the insertion of air in the cylindrical chamber 2. While pushing the lever, pull the piston up, until its incision concurs with the mark of 300 ml. Turn the regulators of the of bottom tap , so as the chamber communicates only with the manometer.
3. Take a look that the pressure gauge readings is O.H., but the pressure of the gas is one atmosphere (1at=1.01325 bars). Each moment the air pressure on the container is p(air)=p(atm)+p(pr.g.) where p(atm) is the atmosphere pressure and p(pr.g.) the reading of the pressure guage. The atmospheric pressure on the surface of the sea is 1atm=1.1325 bar. Therefor when the metallic pressure gauge measures the atmospheric pressure and shows 0 bar, we must correct the readings.
4. On the following table record the pressure gauge reading.
5. By pushing the handle, lower the piston to 280 ml and record again the reading of the pressure gauge.
6. Continue in the same manner with 20ml steps up to 160ml.
7.Repeat the same process for different air temperature which is trapped in the
cylinder.
In order to create defferent temperature conditions, fill the water bath with warm water, pouring it carefully, using a glass for boiling in the black tube which is adapted on the top and of the water bath. Wait until the reading of the digital thermometer is stablised and record the temperature on your work paper. Repeat the measurements (p,v) for different temperature. To come off dofferent temperatures, pour some of the warm water of the water bath into a glass for boiling releasing a little the plastic tube in the top enad and fill in withthe corresponding quantity of cold water on top of it.
In order to have certain quantity of air (same mol) when we take measuments in defferent temperature and be able to compare the charts, we must turn off the tap first (on the 300ml mark), which isolates the container from the environment to the enviromental temperatyre and then pour warm water into the water bath. Therefor the product p.v the second time will be larger than the correspondant of the first measurement. If we first pour warm in the water bath and thn tunr off the top, then a quantity of air will escape the cylinder to the environment due to greater temperature, we will have less mol of air in the
metallic container and the product of p.v will be the same as the first time that we took measuments in environmental temperature.
Work paper (Isothermal variation)
Initial value p(atm)=1,01 bar V(init)=300ml
Measurements
TABLE OF PRESSURE UNITS